Serialization
In the context of the pharmaceutical supply chain, serialization refers to the assignment of a unique, random serial number to each saleable unit, conferring it with a distinctive identity that allows it to be effectively tracked at virtually at any point during its life cycle.
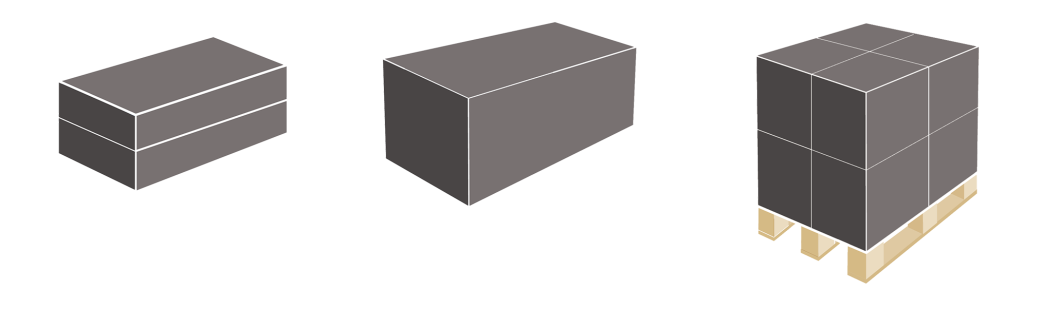
Aggregation
Aggregation refers to the grouping of individually serialized packaging into larger units such as bundles, cases or pallets. These units bear a code that contains information about the serialized packaging within the unit.
Argentina
ANMAT drug traceability system
Administración Nacional de Medicamentos
Serialization
Data Carrier:
Data Element:
GS1-128, DataMatrix, RFID Tag
GTIN
Batch/Lot Number
Expiry Date
Serial Number
Aggregation
Click here to get to the website of the local authority.
Bahrain
Issuing a Track and Trace System for the Supply Chain of Medicine within the Kingdom of Bahrain
SCH Bahrain
Serialization
Data Carrier:
Data Element:
DataMatrix
GTIN
Batch/Lot Number
Expiry Date
Serial Number
Aggregation
Click here to get to the website of the local authority.
Brazil
SNCM “Sistema Nacional de Controle de Medicamentos” Law No. 11.903/2009
ANVISA
Serialization
Data Carrier:
Data Element:
DataMatrix
GTIN
Batch/Lot Number
Expiry Date
Serial Number
Anvisa Registration Number
Aggregation
Data Carrier:
SSCC
SGTIN
Ad-hoc aggregator based on the CNPJ (national company ID)
Serial Number
Anvisa Registration Number
Click here to get to the website of the local authority.
China
CFDA Decree No. 28 “National e-coding system”
CFDA
Serialization
Data Carrier:
Data Element:
Linear/2D Barcode, RFID, HRI
Serial Number
Batch/Lot Number
Expiry Date
Production Date
Aggregation
Data Carrier:
Linear/2D Barcode
RFID
HRI
Click here to get to the website of the local authority.
Egypt
Project on Tracing & Tracking Pharmaceutical Preparations
Inside Arab Republic of Egypt
Aggregation
Data Carrier:
Data Element:
GS1 128 or GS1 DataMatrix
SSCC (AI 00)
Click here to get to the website of the local authority.
European Union
Directive 2011/62/EU
EU Commission
Serialization
Data Carrier:
Data Element:
DataMatrix
Unique product identification number (e. g. GTIN/NTIN/PPN)
Batch/Lot Number
Expiry Date
Serial Number
National reimbursement number (if applicable)
India
Pharmaeutical Export Promotion Counsil of India
Serialization
Data Carrier:
Data Element:
DataMatrix, (or GS1-128), (or GS1-Databar)
GTIN
Batch/Lot Number
Expiry Date
Serial Number
Aggregation
Data Carrier:
Data Element:
GS1-128 (x2)
GTIN
Batch/Lot Number
Expiry Date
Serial number as SSCC (AI 00) – for second code
Click here to get to the website of the local authority.
Indonesia
Regulation Number 33 of 2018
National Agency of Drug and Food Control
Serialization
Data Carrier:
Data Element:
QR Code issued by BPOM, (or own 2D data carrier)
Product Edition Permission Number
Batch/Lot Number
Expiry Date
Serial Number
ID of Production Facility
Click here to get to the website of the local authority.
Iran
Executive guideline No. 660/11873
TTAC
Serialization
Data Carrier:
Data Element:
DataMatrix
GTIN
Batch/Lot Number
Expiry Date
Serial Number
Click here to get to the website of the local authority.
Japan
Implementation Guideline for Barcode Labeling of Prescription Drugs
Ministry of Health, Labour and Welfare (MHLW)
Serialization
Data Carrier:
Data Element:
GS1 DataBar Limited / Stacked
GTIN
Batch/Lot Number
Expiry Date
Serial Number
Aggregation
Data Carrier:
Data Element:
GS1-128
GTIN
Batch/Lot Number
Expiry Date
Serial Number
Click here to get to the website of the local authority.
Jordan
Guidelines of Identification and Bar coding of Medicinal Products for Human Use
JFDA
Serialization
Data Carrier:
Data Element:
DataMatrix
GTIN
Batch/Lot Number
Expiry Date
Aggregation
Data Carrier:
Data Element:
DataMatrix
GTIN
Batch/Lot Number
Expiry Date
Serial Number
Norway
Norwegian Medicines Agency
Serialization
Data Carrier:
Data Element:
Linear/2D Barcode
GTIN
Batch/Lot Number
Expiry Date
Serial Number
Aggregation
Data Carrier:
Data Element:
DataMatrix
GTIN
Batch/Lot Number
Expiry Date
Serial Number
Click here to get to the website of the local authority.
Oman
MH/DGMS/DSS/M/7043
Directorate General of Medicinal Supplies MoH Oman
Serialization
Data Carrier:
Data Element:
DataMatrix
GTIN
Batch/Lot Number
Expiry Date
Serial Number
Click here to get to the website of the local authority.
Pakistan
Ministry of National Health Services, Regulations and Coordination
Serialization
Data Carrier:
Data Element:
DataMatrix
GTIN
Batch/Lot Number
Expiry Date
Serial Number
Product Identification Information
Aggregation
Data Carrier:
Data Element:
DataMatrix
GTIN
Batch/Lot Number
Expiry Date
Serial Number
Product Identification Information
Click here to get to the website of the local authority.
Russia
Federal Law No 488-FZ
Federal Service for Surveillance in Healthcare
Serialization
Data Carrier:
Data Element:
DataMatrix
GTIN
Batch/Lot Number (optional)
Expiry Date (optional)
Serial Number
Aggregation
Data Carrier:
Data Element:
GS1-128
SSCC (AI 00) – for manufacturers and wholesalers
sGTIN (GTIN + Serial Number) – manufacturers only
Identification Code of the tertiary packaging (AI 999): Package extension indicator (N1), wholesale organitazion identifier (N2-N10), control number (N18)
Click here to get to the website of the local authority.
Saudi Arabia
Guidance for the industry on Saudi Drug Code (SDC) and drug barcoding specifiations
Saudi FDA
Serialization
Data Carrier:
Data Element:
DataMatrix
GTIN
Batch/Lot Number
Expiry Date
Serial Number
Aggregation
Data Carrier:
Data Element:
DataMatrix
GTIN
Batch/Lot Number
Expiry Date
Serial Number
Click here to get to the website of the local authority.
South Africa
National Department of Health South Africa
Serialization
Data Carrier:
Data Element:
DataMatrix
GTIN
Batch/Lot Number
Expiry Date
Serial Number
Aggregation
Data Carrier:
Data Element:
GS1-128(or DataMatrix)
GTIN
Batch/Lot Number
Expiry Date
Serial Number
Click here to get to the website of the local authority.
South Korea
Korean Pharmaceuticals Information Service
Ministry for Food and Drug Safety (MFDS)
Serialization
Data Carrier:
Data Element:
DataMatrix, (or GS1-128) ,(or RFID)
KDC (Korean Drug Code)
Batch/Lot Number (optional)
Expiry Date (optional)
Serial Number
Click here to get to the website of the local authority.
Taiwan
Taiwan Food and Drug Administration (TFDA)
Serialization
Data Carrier:
Data Element:
DataMatrix, (or GS1-128),
GTIN
Batch/Lot Number
Expiry Date
Serial Number
Aggregation
Click here to get to the website of the local authority.
Turkey
“Regulation Regarding the Packaging and Labeling of Medicinal Products for Human Use”, Official Gazette 25904
Turkey MoH
Serialization
Data Carrier:
Data Element:
DataMatrix
GTIN
Batch/Lot Number
Expiry Date
Serial Number
Aggregation
Data Carrier:
Data Element:
GS1-128
SSCC (AI 00)
Click here to get to the website of the local authority.
Ukraine
State Service of Ukraine on Medicines and Drug Control
Serialization
Data Carrier:
Data Element:
GS1 bar code,Data Matrix
GTIN
Batch/Lot Number
Expiry Date
Serial Number
Click here to get to the website of the local authority.
USA
Drug Quality Security Act (HR 3204)
US FDA
Serialization
Data Carrier:
Data Element:
DataMatrix
GTIN
Batch/Lot Number
Expiry Date
Serial Number
Aggregation
Click here to get to the website of the local authority.
This website is intended to outline the legislative landscape in terms of pharmaceutical serialization and aggregation. The site raises no claim to completeness. For the most up to date information, please contact the relevant regulatory authority. Last update: 2019/11/04

